Nationwide expansion of Pneumococcal Conjugate Vaccine under the Universal Immunization Programme
Nationwide expansion of Pneumococcal Conjugate Vaccine under the Universal Immunization Programme
Ministry of Health and Family Welfare has launched the nationwide expansion of Pneumococcal Conjugate Vaccine (PCV) under the Universal Immunization Programme (UIP).
Pneumonia
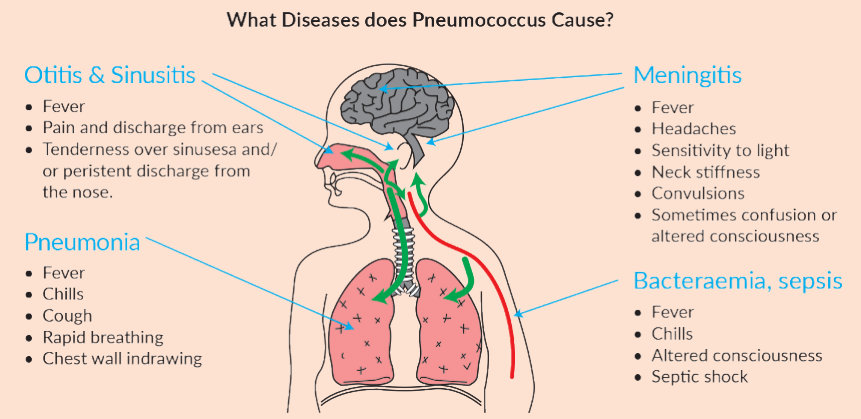
Pneumonia is a leading cause of death among children under-5 years old, globally and in India. Pneumonia caused by Pneumococcus is the most common cause of severe pneumonia in children. In India around 16 percent of deaths in children occur due to Pneumonia. The nationwide rollout of PCV will reduce child mortality by around 60 percent.
Pneumonia is caused by a number of infectious agents, including viruses, bacteria and fungi. The most common are:
- Streptococcus pneumoniae – the most common cause of bacterial pneumonia in children;
- Hib – the second common cause of bacterial pneumonia;
- Respiratory syncytial virus – is the most common viral cause of pneumonia;
- Pneumocystis jiroveci – responsible for at least one quarter of all pneumonia deaths in human immunodeficiency virus (HIV)-infected infants.
 Pneumococcal infection is transmitted by direct contact with respiratory secretions from patients and healthy carriers
Pneumococcal infection is transmitted by direct contact with respiratory secretions from patients and healthy carriers
About UIP
Universal Immunization Programme (UIP) is one of the largest public health programmes targeting close to 26.7 million newborns and 29 million pregnant women annually.
Under UIP, immunization is being provided free of cost against 12 vaccine preventable diseases:
- Nationally against 10 diseases - Diphtheria, Pertussis, Tetanus, Polio, Measles, Rubella, severe form of Childhood Tuberculosis, Rotavirus diarrhea, Hepatitis B and Meningitis & Pneumonia caused by Haemophilus Influenzae type B
- Sub-nationally against 2 diseases - Pneumococcal Pneumonia and Japanese Encephalitis; of which Pneumococcal Conjugate vaccine is nationally expanded today, while JE vaccine is provided only in endemic districts.
Currently, three vaccines have the potential to significantly reduce childhood mortality from and related to pneumonia: PCV, Hib-containing pentavalent vaccine and measles vaccine.
Pneumococcal conjugate vaccines (PCV)
 The National Technical Advisory Group for Immunization (NTAGI) in 2015, based on the available documents regarding product specifications, projected availability, and operational feasibility including multi-dose presentation and compliance with open vial policy, recommended PCV13 (4-dose vial) as the preferred vaccine type for introduction in the UIP. The PCV13 4-dose vial is WHO prequalified.
The National Technical Advisory Group for Immunization (NTAGI) in 2015, based on the available documents regarding product specifications, projected availability, and operational feasibility including multi-dose presentation and compliance with open vial policy, recommended PCV13 (4-dose vial) as the preferred vaccine type for introduction in the UIP. The PCV13 4-dose vial is WHO prequalified.
Dosage
The dose of the PCV vaccine is 0.5 ml and to be administered by intramuscular injection in the anterolateral aspect of the right mid thigh of infants. If multiple injections must be given in the same thigh, the distance between the two injections should be at least 2.5 cm (1 inch).
Schedule
PCV will be administered in three doses (2 primary and 1 booster) at 6 weeks, 14 weeks and 9 months of age as part of routine immunization.
- The first dose, PCV1, will be administered at 6 weeks of age with the first dose of pentavalent vaccine, oral polio vaccine (OPV), fractional-dose IPV1 and
rotavirus vaccine. - The second dose, PCV2, will be given at 14 weeks of age, with the third dose of pentavalent vaccine, oral polio vaccine, fractional-dose IPV2 and rotavirus
vaccine - The PCV booster dose will be administered at 9 months of age with the first dose of measles-rubella (MR) vaccine and first dose of Japanese Encephalitis
(JE) vaccine (in endemic districts).
The two primary doses and one booster dose of PCV should be given during the first year of life. If the doses are delayed within the first year of life, delayed
doses must be separated by a minimum interval of at least 8 week s, to be given at the next scheduled immunization visit.
In delayed cases beyond 1 year of age, due doses can be given to a child only if a child has received at least one dose of PCV before his/her first birthday.
To view the operational guidelines for PCV in India, click here.
Source : Ministry of Health and Family Welfare
Last Modified : 8/1/2023
This topic provides information about Mission Rout...
This topic provides information related to Nationa...
A digital platform to capture all vaccination acti...
The topic covers various aspects about immunizatio...
